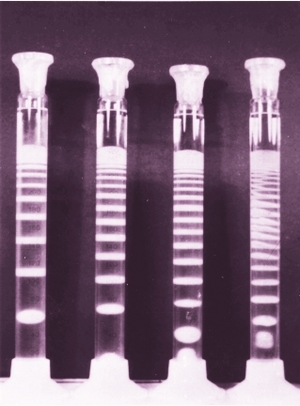
How to make Liesegang patterns
It is relatively easy to make some nice Liesegang patterns. One needs only some gelatine or other gel-forming material, several test tubes or Petri dishes, and small amount of reactants that form an insoluble reaction product (precipitate) with each other.
In a standard Liesegang experiment a soluble electrolyte at relatively low concentration is placed in a container, to which a gel forming material is added. (The gel is produced using this dilute solution instead fo water.)
After the gelation process completed, a second solution usually at substantially higher concentration is poured onto the top of the gel. If the two electrolytes react with each other and form a weakly soluble material (precipitate), a series of precipitate zones will form in the gel matrix.
Certainly there are many appropriate eletrolyte pairs, however there are some systems that produce Liesegang patterns more easily.

Using chemically crosslinked poly-vinyl-alcohol gel the inner electrolyte can be for example magnesium-sulphate, while the outer one concentrated ammonium-hydroxide. These will form white magnesium-hidroxide precipitate.
Liesegang experiments can be performed in two essentially different arragements.
One can use a simple test tube in which case the precipitate will form bands perpendicularly to the direction of diffusion.
However, a similar pattern can be produced, if we use a gel sheet. Such a sheet can be produced if we pour the viscous solution of the gelling material onto a glass plate. In this case after the gel set, we put a drop of concentrated solution of the outer electrolyte onto the surface of the gel sheet. A "more sophisticated" method is if we cut a cylindrical hole into the sheet and use this as the container of the invading electrolyte.

In this case the precipitate will form rings around the droplet or hole. Liesegang himself has performed such a two dimensional experiment in 1896.
Maybe it is important to mention that some workers have found similar patterning in the absence of any gel like material. Precipitate zones can be formed for example in capillary tubes in pure water, or in gas phase, when HCl and NH3 diffuses into each other in a porous media (silica or active carbon).
Because of these findings, it has been widely accepted that the role of the gel is only to prevent sedimentation of the precipitate, and convection of the electrolytes.

Recipe: Liesegang pattern from silver-chromate in gelatine
Put 4 g dry gelatine and 0.12 g potassium-bichromate into 120 ml warm distilled water. Fill half a test tube with this mixture and wait until the completion of gelation.
After this pour 5 ml of 8 m/m % silver-nitrate solution onto the gel. After 1-2 days (!) nice silver-bichromate zones will form.
The same solutions can be used to produce Liesegang rings in a gel sheet. In this case before the end of galtion pour the viscous solution onto a glas plate or into a Petri dish. After this put a droplet of silver-nitrate solution onti the gel surface and cover the system in order to avoid desiccation.
Recipe: magnesium-hydroxide pattern in gelatine
Make a solution of magnesium-chloride with a concentration between 0.01 and 0.2 mol/l. Give to this solution dry gelatine up to 3 m/m %. Wait half an hour and put the solution onto a steam bath of 60-70 oC and wait again until the complete solubilization of geltine.
Pour the solution into test tubes and after the gel set pour 4 ml of concentrated ammonium-hydroxide solution onto it. Close the tubes hermetically, because ammonia is very volatile.